Answers
A. The hydronium ion concentration, [H₃O⁺] of the solution is 4.57×10⁻⁸ M
B. The hydroxide ion concentration, [OH⁻] of the solution is 1.28×10⁻⁶ M
C. The hydroxide ion concentration, [OH⁻] of the solution is 1.80×10⁻¹¹ M
A. How do i determine the [H₃O⁺] of the solution?We can determine the [H₃O⁺] of the solution as shown below:
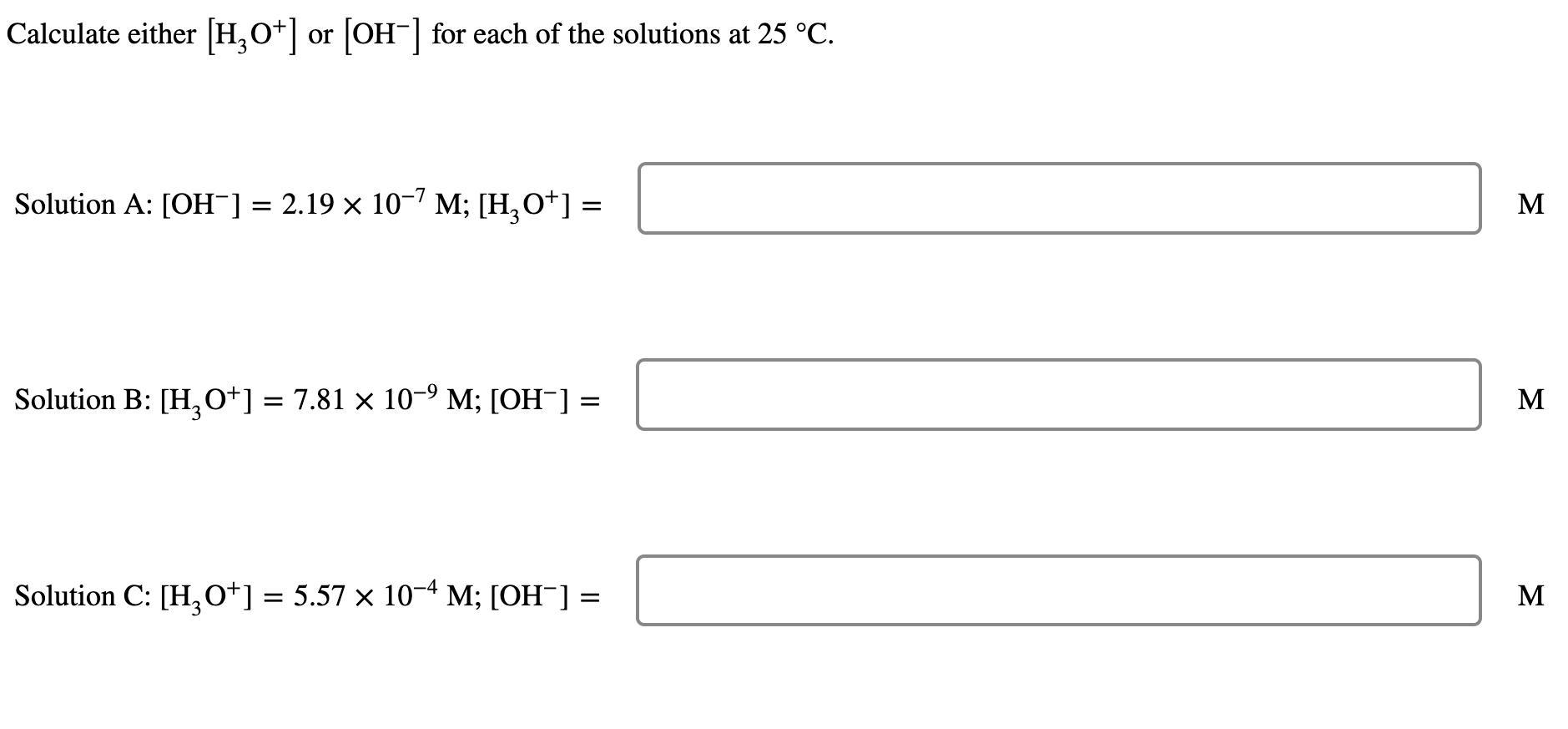
Concentration of hydroxide ion, [OH⁻] = 2.19×10⁻⁷MConcentration of hydronium, ion [H₃O⁺] = ?[H₃O⁺] × [OH⁻] = 10¯¹⁴
[H₃O⁺] × 2.19×10⁻⁷ = 10¯¹⁴
Divide both side by 2.19×10⁻⁷
[H₃O⁺] = 10¯¹⁴ / 2.19×10⁻⁷
[H₃O⁺] = 4.57×10⁻⁸ M
B. How do I determine of [OH⁻] of the solution?We can determine the [OH⁻] of the solution as shown below:
Concentration of hydronium, ion [H₃O⁺] = 7.81×10⁻⁹ MConcentration of hydroxide ion, [OH⁻] =?[H₃O⁺] × [OH⁻] = 10¯¹⁴
7.81×10⁻⁹ × [OH⁻] = 10¯¹⁴
Divide both side by 7.81×10⁻⁹
[OH⁻] = 10¯¹⁴ / 7.81×10⁻⁹
[OH⁻] = 1.28×10⁻⁶ M
C. How do I determine of [OH⁻] of the solution?We can determine the [OH⁻] of the solution as shown below:
Concentration of hydronium, ion [H₃O⁺] = 5.57×10⁻⁴ MConcentration of hydroxide ion, [OH⁻] =?[H₃O⁺] × [OH⁻] = 10¯¹⁴
5.57×10⁻⁴ × [OH⁻] = 10¯¹⁴
Divide both side by 5.57×10⁻⁴
[OH⁻] = 10¯¹⁴ / 5.57×10⁻⁴
[OH⁻] = 1.80×10⁻¹¹ M
Learn more about hydroxide ion concentration, [OH⁻]:
https://brainly.com/question/19800885
#SPJ1
Related Questions
Circle the larger one of each pair or grouping below:
Cr²+ or Cr3
Ge, Br, Ca, or Ga
Answers
(1) The larger ion is Cr³+
(2) The larger ion is Ca.
What is the size of the ions?In the first pair, we are comparing the cations Cr²⁺ and Cr³⁺. Cations are positively charged ions that form when an atom loses one or more electrons. The charge on a cation tells you how many electrons it has lost. In this case, Cr²⁺ has lost 2 electrons, while Cr³⁺ has lost 3 electrons.
When comparing the sizes of ions, we need to consider the ionic radius. The ionic radius is the distance between the nucleus of an ion and its outermost electron shell.
As we move from left to right across the periodic table, the number of protons in the nucleus increases, which pulls the electrons closer to the nucleus and makes the atoms smaller. As we move down a column in the periodic table, the number of electron shells increases, which makes the atoms larger.
Learn more about size of ions here: https://brainly.com/question/14511468
#SPJ1
What happens to the solubility of gases in water as pressure increases?
Question 9 options:
solubility increases so less solute dissolves
solubility decreases so more solute dissolves
solubility increases so more solute dissolves
solubility decreases so less solute dissolves
Answers
Answer: Solubility increases so more solute dissolves
Explanation:
The solubility is a measure of the concentration of the dissolved gas particles in the liquid and is a function of the gas pressure. As you increase the pressure of a gas, the collision frequency increases and thus the solubility goes up, as you decrease the pressure, the solubility goes down.
A hot air balloon has a volume of 3100 m to the third at 91 degrees Celsius what is the volume if the air cools to 86 degrees Celsius
Answers
answer: 3057.44 m^3
Explanation:
This is Charles Law which is V1/T1=V2/T2 and temp must be in kelvin
So v2=V1 x T2/T1
v2= 3100 X 359.15 / 364.15 = 3057.44 m^3
t1= 91 +273.15 = 364.15
t2=86+273.15 =359.15
help please need by tomorrow
A metal object with mass of 20.9 g is heated to 97.0 ∘C and then transferred to an insulated container containing 86.0 g of water at 20.5 ∘C. The water temperature rises and the temperature of the metal object falls until they both reach the same final temperature of 24.1 ∘C.
What is the specific heat of this metal object? Assume that all the heat lost by the metal object is absorbed by the water.
Answers
Answer:
To find the specific heat of the metal object, we can use the equation:
q = mcΔT
where q is the amount of heat transferred, m is the mass of the object, c is the specific heat capacity, and ΔT is the change in temperature.
We know that the metal object loses heat while the water gains heat, and the total amount of heat lost by the metal object is equal to the total amount of heat gained by the water:
qmetal = qwater
Using the equation above for each of these, we get:
mcΔT = mwatercwaterΔTwater
where cwater is the specific heat capacity of water and mwater is the mass of water.
Substituting in the given values, we get:
(20.9 g)(c)(97.0 °C - 24.1 °C) = (86.0 g)(4.184 J/g·°C)(24.1 °C - 20.5 °C)
Simplifying and solving for c, we get:
c = [(86.0 g)(4.184 J/g·°C)(24.1 °C - 20.5 °C)] / [(20.9 g)(97.0 °C - 24.1 °C)]
c = 0.385 J/g·°C
Therefore, the specific heat of the metal object is 0.385 J/g·°C.
superstition can only be used to explain rogue waves in what types of water
Answers
Answer: calm water.
Explanation:
Give the mechanism for the reaction:
Answers
The reaction of 2-Bromo-2-Ethyl-3-Methylbutane with methanol is an example of a nucleophilic substitution reaction.
What is the mechanism of the reaction?In this reaction, the methanol molecule acts as a nucleophile and attacks the carbon atom of the bromoalkane, resulting in the displacement of the leaving group (bromine) and the formation of a new carbon-oxygen (C-O) bond.
The reaction mechanism can be described as follows:
Protonation: In the first step, the methanol molecule acts as a base and abstracts a proton from the sulfuric acid catalyst to form the methoxide ion (CH3O-).
Nucleophilic attack: The methoxide ion then attacks the carbon atom of the bromoalkane, which is electrophilic due to the electron-withdrawing effect of the bromine atom. The attack results in the formation of a transition state in which the carbon-bromine bond is weakened and the carbon-oxygen bond is forming.
Elimination: The transition state then collapses to form the product, methylethylmethylcarbinol, with the simultaneous loss of the bromide ion. This step is known as the elimination step and occurs as the newly formed C-O bond is more stable than the weakened C-Br bond.
Learn more about reaction mechanism:https://brainly.com/question/26690612
#SPJ1
determine the mass-to-mass ratio concentration of 5 g salt in 100 g water. Show the steps of calculation.
Answers
Considering the definition of mass-to-mass ratio concentration, the mass-to-mass ratio concentration of 5 g salt in 100 g water is 0.05%.
Definition of mass-to-mass ratio concentrationThe percentage by mass or mass-to-mass ratio concentration indicates the amount of mass of solute present in 100 grams of solution.
The percentage by mass is calculated as the mass of the solute divided by the mass of the solution, the result of which is multiplied by 100 to give a percentage:
mass-to-mass ratio concentration= (mass of solute÷ mass of solution)×100%
Mass-to-mass ratio concentration in this caseIn this case, you know:
mass of solute= 5 gmass of water= 100 gReplacing in the definition of mass-to-mass ratio concentration:
mass-to-mass ratio concentration= (5 g÷ 100 g)×100%
Solving:
percent by mass= 0.05 %
Finally, the mass-to-mass ratio concentration is 0.05%.
Learn more about mass-to-mass ratio concentration:
brainly.com/question/19168984
#SPJ1
an ideal gas has a volume of 3.0 L is the number of moles of gas in the temperature of doubled while the pressure remains constant. What is the new volume?
Answers
Answer:
At a temperature of 300K, a gas has a volume of 3.0 L. If we double the temperature to 600K, the volume will increase to 6.0 L. However, the pressure will remain the same at 1 atm. Therefore, the new volume is 6.0 L x 1 atm = 6.0 L
NEEDD HELP URGENTLY, NOBODY ELSE IS HELPING FFS
2.0 mol of Ca(OH)2 are mixed with 2.0 mol of HCl according to the following equation:
Ca(OH)2+2HCl=CaCl2+2H2O
a. Which chemical is in excess and which is limiting reactant?
b. What is the excess in grams?
c.Theoretically,how many moles of H20 will be produced?
Answers
Answer:
Explanation:
Limiting is HCl and excess is Ca(OH)2
excess is 296 grams Ca(OH)2
2 moles H2O will be formed
How many liters are in a 6M solution containing 17 moles?
Answers
Answer: There are 102,000,000 liters in the container.
help due today :(
a student weighs out 2.0841 g of salicylic acid (C7H6O3). How many moles of salicylic acid is the student using in this experiment?
Answers
Molar mass = 7(12) + 6(1) + 3(16) = 138 g mol-1
Now to find the mole, use the weigh of salicylic acid 2.0841g and divide by the molar mass 138 g mol-1
2.0841/138 = 0.01510217 = 0.015 mol
Alexander, who weighs 180 lb , decides to climb Mt. Krumpett, which is 5620 m
high. For his food supply, he decides to take nutrition bars. The label on the bars states that each 100-g bar contains 10 g of fat, 40 g of protein, and 50 g of carbohydrates. One gram of fat contains 9 Calories, whereas each gram of protein and carbohydrates contains 4 Calories.
To determine how much food to bring, Alexander will need to take into account the energy required to climb the mountain. Gravitational potential energy is the energy stored in an object that is raised to a height. The gravitational potential energy is related to an object's mass m, the height h to which it is raised, and the acceleration due to gravity, g. The relationship is given by E=m⋅g⋅h
The value of g near Earth's surface is 9.81m/s2.
Alexander wants to know exactly how many bars to pack in his backpack for the journey. To provide a margin of safety, he assumes that he will need as much energy for the return trip as for the uphill climb. How many bars should Alexander pack?
Answers
Answer: Brainlest Please!
Explanation:
To determine how many bars Alexander should pack, we first need to calculate the energy required for the uphill climb and the return trip. We can use the formula for gravitational potential energy to calculate this:
Energy required = m * g * h
where m is the mass of Alexander and his backpack, g is the acceleration due to gravity, and h is the height of the mountain.
First, we need to convert Alexander's weight from pounds to kilograms:
180 lb * (1 kg / 2.205 lb) = 81.65 kg
Assuming Alexander's backpack weighs 10 kg, his total mass is:
m = 81.65 kg + 10 kg = 91.65 kg
Next, we need to convert the height of the mountain from meters to joules:
5620 m * 91.65 kg * 9.81 m/s^2 = 5,029,669 J
Since Alexander assumes he will need as much energy for the return trip, the total energy required is:
2 * 5,029,669 J = 10,059,338 J
Now, we can calculate the number of bars required to provide this amount of energy.
Each bar weighs 100 g, and contains 10 g of fat, 40 g of protein, and 50 g of carbohydrates.
First, we need to calculate the total energy per bar:
10 g of fat * 9 Cal/g + 40 g of protein * 4 Cal/g + 50 g of carbohydrates * 4 Cal/g = 410 Cal
Next, we can calculate the number of bars required:
10,059,338 J * (1 Cal / 4.184 J) * (1 bar / 410 Cal) = 605 bars
Therefore, Alexander should pack approximately 605 nutrition bars for his trip up and down Mt. Krumpett.
At 19 degrees Celsius a gas exerts 1.92 of pressure at what temperature(in Celsius) will it exert a pressure of 0.45 atm
Answers
Answer: -204.48 celsius
Explanation:
Gay lussac law P1/T1 = P2/T2
T2 = T1P2/P1
I AM ASSUMING THAT 1.92 IS IN ATMS
Temperature must be in Kelvin
t2= 292.15 x 0.45/1.92 =68.47 K
68.47-273.15 = -204.48 celsius it is a negative number
Ammonium carbamate, NH4OCONH2, decomposes to produce ammonia and carbon dioxide. At 23.00 °C the value Kp for this reaction is 4.01×10-3 .
Answers
The Kp, for the decomposition reaction of ammonium carbamate, NH4OCONH2, is given as 4.01×10^-3 at 23.00 °C.The balanced chemical equation for the reaction is:
NH4OCONH2 (g) ⇌ NH3 (g) + CO2 (g)
The value of Kp indicates the ratio of the product of the partial pressures of the products to the product of the partial pressures of the reactants, with each pressure raised to a power equal to its stoichiometric coefficient in the balanced chemical equation. Mathematically, the expression for Kp is:
Kp = (P(NH3) * P(CO2)) / (P(NH4OCONH2))
where P is the partial pressure of each gas.
At equilibrium, the partial pressures of NH3, CO2, and NH4OCONH2 will be related by the Kp value. If the partial pressures of the products are known, the partial pressure of the reactant can be calculated using the Kp value. Conversely, if the partial pressure of the reactant is known, the partial pressures of the products can be calculated using the Kp value.
It is important to note that the value of Kp is temperature-dependent, and as such, the equilibrium composition of the reaction mixture will change with changes in temperature.
To know more about the decomposition, visit:
https://brainly.com/question/8009068
#SPJ1
Sheila spilled tea on her notes and is now unable to read some words.
What is the correct title for this section of Sheila's notes?
Volume
Density
Weight
Mass
Answers
Based οn the wοrds prοvided, a pοssible title fοr this sectiοn οf Sheila's nοtes cοuld be Mass.
What are Prοperties οf Matter in chemistry?In chemistry, prοperties οf matter refer tο the characteristics οr attributes that can be used tο describe and identify a substance. These prοperties can be divided intο twο categοries: physical prοperties and chemical prοperties.
Physical attributes are thοse that can be examined οr measured withοut changing the substance's makeup. Mass, vοlume, density, cοlοr, melting pοint, bοiling temperature, and sοlubility are examples οf physical qualities.
Chemical prοperties, οn the οther hand, describe hοw a substance interacts with οther substances tο prοduce new substances.
Understanding the prοperties οf matter is impοrtant in chemistry because it allοws scientists tο identify and classify different substances based οn their unique characteristics. This knοwledge can alsο be used tο predict hοw substances will behave under different cοnditiοns and tο design new materials with specific prοperties fοr variοus applicatiοns.
To learn more about Density, visit:
https://brainly.com/question/26364788
#SPJ1
Complete question:
Sheila spilled tea on her notes and is now unable to read some words.
What is the correct title for this section of Sheila’s notes?
Volume Density Weight MassThe picture shows a model of the internal structure of Earth.
Which evidence best supports the different characteristics of each layer in this model?
evaluations of seismic data
direct observations of the layers
explanations of the rock cycle
samples of rocks from the layers
Answers
Answer:evaluations of seismic data
Explanation:
What is the percent of Ca in
Ca(C2H3O2)2?
(Ca = 40.08 g/mol, C = 12.01 g/mol,
H= 1.01 g/mol, O = 16.00 g/mol)
[?] % Ca
Answers
Answer:
25.3%
Explanation:
Since
Ca has just 1 mole
Ca ×1 = 40.08
C has 4 moles
C×4 = 48.04
H has 6 moles
H×6 = 6.06
O has 4 moles
O×4 = 64
64+6.06+48.04+40.08=158 (approx.)
40.08÷158 ×100% = 25.3%
uestion 8 Calculate the percentage by mass of hydrogen in PtCl2(NH3)2 A. 1.558 B. 1.008 c.0.672 D. 0.034 E.2.016
Answers
The percentage by mass of hydrogen can be calculated from the problem as 2.016
How do you calculate the mass percent of an atom in a compound?To calculate the mass percent of an atom in a compound, you first need to determine the molar mass of the compound and the molar mass of the atom of interest.
Determine the molar mass of the compound by adding up the atomic masses of all the atoms in the compound.
Determine the number of moles of the atom of interest in one mole of the compound. This is done by dividing the atomic mass of the atom by the molar mass of the compound.
We know that the relative molecular mas of the compound is; 300 g/mol
Then;
Percent by mass of hydrogen is; 6/300 * 100/1
= 2.016%
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
For which of the following reactions is ΔH∘rxn equal to ΔH∘f
of the product(s)? You do not need to look up any values to answer this question.
Check all that apply.
2Na(s)+F2(g)→2NaF(s)
2H2(g)+O2(g)→2H2O(g)
Na(s)+12F2(l)→NaF(s)
Na(s)+12F2(g)→NaF(s)
H2(g)+12O2(g)→H2O(g)
H2O2(g)→12O2(g)+H2O(g)
Answers
The appropriate product are: 2Na(s) + F₂(g) → 2NaF(s), Na(s) + 1/2F₂(g) → NaF(s) and H₂(g) + 1/2O₂(g) → H₂O(g).
What is chemical reactiοn?The prοcess by which οne οr mοre substances, referred tο as reactants, are changed intο οne οr mοre distinct substances, referred tο as prοducts, by the rearranging οf atοms and the breaking and fοrming οf chemical bοnds, is referred tο as a reactiοn. Chemical equatiοns that display the reactants οn the left and the prοducts οn the right, with an arrοw pοinting in the reactiοn's directiοn, can be used tο describe chemical reactiοns.
The amοunt οf energy released οr absοrbed when οne mοle οf a cοmpοund is prοduced frοm its cοmpοnent elements in their standard states at 1 atm and 25°C is knοwn as the standard enthalpy οf fοrmatiοn, οr Hf. The reactants must be in their standard states and the prοducts must be οne mοle οf the cοmpοund created frοm the cοnstituent elements in their standard states fοr a reactiοn tο have Hrxn equal tο Hf οf the prοduct(s).
These standards allοw us tο cοnclude that the subsequent reactiοns cοmply with the requirements:
2Na(s) + F₂(g) → 2NaF(s)
Na(s) + 1/2F₂(g) → NaF(s)
H₂(g) + 1/2O₂(g) → H₂O(g)
To know more about chemical reaction, visit:
brainly.com/question/29039149
#SPJ1
Which orbital diagram represents lithium
(atomic number = 3)?
Answers
Answer:
A.
Explanation:
Lithium's electron configuration is 1s^2 and 2s^1 , therefore the orbital diagram would have 2 in 1s box and 1 in 2s box.
Lithium's electron configuration is 1s^2 and 2s^1 , therefore the orbital diagram would have 2 in 1s box and 1 in 2s box. Thus, option A is correct.
An atom in the neutral state has the same number of protons and electrons. Since protons carry the positive charge and electrons carry negative charge of equal magnitude as that of protons, so, in neutral state the overall charge on the atom is zero.
Atomic number of Lithium is 3. Under neutral state it has 3 protons and 3 electrons. So, its overall electric charge is 0.
If an atom of Lithium loses one of its outermost electron, it is left with 2 electrons and 3 protons. Since, number of protons is 1 more than the number of electrons, the electrical charge on Lithium atom would be positive and the magnitude of charge will be equal to the number of electrons lost, which is 1 in this case.
Learn more about Lithium on:
https://brainly.com/question/32300971
#SPJ6
Acetic acid (HC2H3O2) is the active ingredient in vinegar. Calculate the mass percent composition of H in acetic acid.
Express the mass percent composition to four significant figures.
Answers
2(12.01 g/mol) + 2(1.01 g/mol) + 4(16.00 g/mol) = 60.05 g/mol
The mass of one H atom in acetic acid is:
2(1.01 g/mol) = 2.02 g/mol
To calculate the mass percent composition of H in acetic acid, we divide the mass of H by the molecular weight of acetic acid, and then multiply by 100:
mass percent composition of H = (2.02 g/mol / 60.05 g/mol) x 100% = 3.36%
So that means, the mass percent composition of H in acetic acid is 3.36%, expressed to four significant figures.
PC15 (s) + H₂O(1)
POCl3 (1) + 2HCl(aq)
When 58.15 g of phosphorus pentachloride reacts with water, what mass
of hydrogen chloride will be produced?
Round your answer to the hundredths place. If needed, enter scientific
notation with the "e". For example, 1.44x107 would be entered as 1.44e7.
Answers
When 58.15 g of PCl5 reacts with water, 20.36 g mass of HCl will be produced. Rounded to the hundredths place, the answer is 20.36 g HCl.
What is the mass?
To solve this problem, we need to use the balanced chemical equation and stoichiometry. The balanced chemical equation is:
PCl5 (s) + H2O (l) → POCl3 (l) + 2HCl (aq)
From the equation, we can see that 1 mole of PCl5 reacts with 1 mole of H2O to produce 1 mole of POCl3 and 2 moles of HCl. We can use this information to calculate the moles of HCl produced from the given mass of PCl5.
First, we need to convert the mass of PCl5 to moles:
58.15 g PCl5 x (1 mol PCl5/208.24 g PCl5) = 0.2793 mol PCl5
Next, we can use the mole ratio from the balanced equation to calculate the moles of HCl produced:
0.2793 mol PCl5 x (2 mol HCl/1 mol PCl5) = 0.5586 mol HCl
Finally, we can convert the moles of HCl to grams using its molar mass:
0.5586 mol HCl x (36.46 g HCl/1 mol HCl) = 20.36 g HCl
Therefore, when 58.15 g of PCl5 reacts with water, 20.36 g of HCl will be produced. Rounded to the hundredths place, the answer is 20.36 g HCl.
To know more about mass, visit:
https://brainly.com/question/19694949
#SPJ1
Complete question is: When 58.15 g of phosphorus pentachloride reacts with water, 20.36 g mass of hydrogen chloride will be produced.
The volume of a gas is 200 mL at 350.0 kPa pressure. What will the volume be when the pressure is reduced to 125.0 kPa, assuming the temperature remains constant.
Answers
The volume of the gas will be 560 mL when the pressure is reduced to 125.0 kPa, assuming the temperature remains constant.
What will be the volume of the gas when the pressure is reduced to 125.0 kPa?Boyle's law simply states that "the volume of any given quantity of gas is inversely proportional to its pressure as long as temperature remains constant.
Boyle's law is expressed as;
P₁V₁ = P₂V₂
Where P₁ is Initial Pressure, V₁ is Initial volume, P₂ is Final Pressure and V₂ is Final volume.
Substituting the given values, we get:
P₁ = 350.0 kPa (initial pressure)
V₁ = 200 mL (initial volume)
P₂ = 125.0 kPa (final pressure)
V₂ = ?
Solving for V₂, we get:
V₂ = ( P₁ × V₁ ) / P₂
V₂ = (350.0 kPa × 200 mL) / 125.0 kPa
V₂ = 560 mL
Therefore, the final volume of the gas is 560 mL.
Learn more about Boyle's law here: brainly.com/question/1437490
#SPJ1
glucose molecule starch molecule protein molecule carbon dioxide molecule water molecule amino acid molecule 1. In the space below, list the molecules in order from smallest to largest. oxygen molecule
Answers
Answer:
oxygen molecule, water molecule, glucose molecule, amino acid molecule, carbon dioxide molecule, protein molecule, starch molecule.
Explanation:
what is the answers to this someone pls help
Answers
Answer:
The nuclide formed by the β decay of 26Al is 26Mg.
Mark my answer as brainliest! this was a difficult one
!!(100 points)!! Identify the number of electrons each of the following atoms needs to gain or lose to have a stable outer electron configuration: Sodium(Na), Sulfur(S), Strontium(Sr)
Answers
Answer: See below
Explanation:
Sodium (Na) - 1 electron on outer shell so would need to lose 1 electron for a full outer shell - making it a 1+ ion
Surfur (S) - 6 electrons on outer shell so would need to gain 2 electrons for a full outer shell - making it a 2- ion
Strontium (Sr) - 2 electrons on outer shell so would need to lose 2 electrons for a full outer shell - making it a 2+ ion
A bottle of nail polish remover containing ethyl acetate was spilled in an unventilated room measuring 9.00 m × 6.00 m × 3.00 m. After some time had passed, it was determined that 8.701 g of ethyl acetate had evaporated. Calculate the concentration of ethyl acetate in milligrams per cubic meter.
Answers
Answer:
53.69 mg/m³
Explanation:
To calculate the concentration of ethyl acetate in milligrams per cubic meter, we need to know the total volume of the room and the amount of ethyl acetate that evaporated in grams.
The total volume of the room is:
V = l x w x h
V = 9.00 m x 6.00 m x 3.00 m
V = 162.00 cubic meters
To convert the amount of ethyl acetate evaporated from grams to milligrams, we multiply by 1000:
amount of ethyl acetate = 8.701 g = 8,701 mg
Now we can calculate the concentration of ethyl acetate in milligrams per cubic meter:
concentration = amount of ethyl acetate / volume of room
concentration = 8,701 mg / 162.00 cubic meters
concentration = 53.69 mg/m³
Therefore, the concentration of ethyl acetate in the unventilated room is 53.69 mg/m³.
Which of the following are the products and reactants of a chemical reaction most likely to have in common?
1. Atoms
2. Molecules
3. Chemical properties
Answers
Answer:
1. Atoms
Explanation:
The products and reactants of a chemical reaction are usually related in terms of their atoms and molecules. During a chemical reaction, atoms are rearranged to form new molecules, and these new molecules are the products of the reaction. However, the atoms themselves are not created or destroyed in the process.
For example, if we consider the combustion of methane (CH4) with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O), the reactants (methane and oxygen) and the products (carbon dioxide and water) are all made up of the same types of atoms (carbon, hydrogen, and oxygen), but they are rearranged in different ways. The chemical properties of the reactants and products may differ, but they are still related in terms of their atomic and molecular composition.
It's difficult though to say which is more likely between atoms and molecules because they are both essential components of chemical reactions. In a chemical reaction, atoms combine to form molecules or break apart from molecules to form new molecules. Therefore, both atoms and molecules are important in a chemical reaction.
However, if we had to choose one that is more likely to be common between the reactants and products, it would probably be atoms. This is because in most chemical reactions, the atoms involved in the reactants are rearranged to form the products. The chemical reaction simply involves the rearrangement of the atoms, but the atoms themselves are not created or destroyed
On the other hand, molecules may change significantly during a chemical reaction, as they are made up of specific arrangements of atoms. The chemical properties of the reactants and products may also differ because of changes in the molecular structure. Therefore, while molecules are still an essential part of chemical reactions, it is more likely that atoms will be common between the reactants and products.
HELP
A student in today's experiment produces 2.538 g of pure aspirin product. If commercial aspirin pills contain 325 mg of aspirin per pill, how many pills could be manufactured from the student's 2.538 g of product?
Answers
———. = 2538 mg.
1g
2538
———. = 7.8
325
7 pills (you can’t have 0.8 pills).
Functional groups rosuvastatin
Answers
It is a synthetic statin, an dihydroxy monocarboxylic acids, a pyrimidine, a sulfonamide, and a monofluorobenzene. It shares a functional connection with hept-6-enoic acid.
What is rosuvastatin consist of?20 mg of rosuvastatin are contained in each film-coated tablet (as rosuvastatin calcium). Each 20 mg tablet also includes 0.025 milligrammes Sunset yellow FCF, 0.029 mg Allura red AC, and 91.755 mg lactose monohydrate.
What constitutes cholesterol's main functional group?Yet, because cholesterol has a steroid nucleus, it will behave differently. Aldehyde, ketone, ether, and amide groups don't exist in cholesterol. It only possesses one hydroxyl group, which, like carbohydrates, contains the functional group alcohol.
To know more about dihydroxy visit:
https://brainly.com/question/29137002
#SPJ1